Noble Gas Notation For Oxygen
Electron Configurations
The content that follows is the substance of Full general Chemistry Lecture 26. In this lecture we continue the word of Breakthrough Numbers and their use in Electron Configurations as well as the human relationship of electron configuration to the periodic properties of the elements.
Electron Configuration
Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral cantlet has a number of electrons equal to its number of protons. What nosotros will practise now is identify those electrons into an system effectually the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain:
So based on what we know about the quantum numbers and using the chart above, yous need 2 electrons to fill an southward orbital, half-dozen electrons to make full a p orbital, x electrons to fill a d orbital and 14 electrons to fill up the f orbital. Merely what nosotros oasis't discussed is how these orbitals become filled...the order of fill.
Society of Make full
The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first. Simply like the breakthrough numbers themselves this order was determined by calculation and is summarized by the following chart:
or y'all can simply utilise the periodic tabular array:

How to Write an Electron Configuration
The symbols used for writing the electron configuration outset with the trounce number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital.
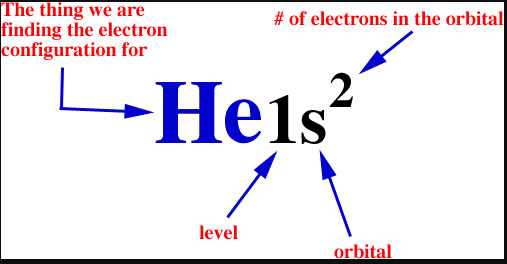
For example:
Looking at the periodic tabular array, you lot tin can meet that Oxygen has eight electrons. Based on the order of fill higher up, these 8 electrons would fill in the following society 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1sii2s22p4 .
Special Cases
Configurations of ions present a special example of electron configuration and likewise demonstrate the reason for the formation of those ions in the start place.
If you need to write the full electron configuration for an anion, then you are merely adding additional electrons and the configuration is merely continued.
For example, we know that Oxygen e'er forms 2- ions when it makes an ion. This would add 2 electrons to its normal configuration making the new configuration: O2- 1s22s22p6 . With 10 electrons you should note that oxygen'south electron configuration is now exactly the same as Neon's. We talked about the fact that ions form because they can get more stable with the gain or loss of electrons to go like the noble gases and now you can really run into how they become the same.
The electron configurations for Cations are also made based on the number of electrons simply there is a slight difference in the way they are configured. Offset y'all should write their normal electron configuration and then when you remove electrons y'all take to have them from the outermost shell. Annotation that this is non ever the same way they were added.
Here is an example of what I mean:
Iron has 26 electrons so its normal electron configuration would exist: Fe 1s22s22p63s23pvi4stwo3d6
When we make a three+ ion for Iron, we need to take the electrons from the outermost shell commencement so that would be the 4s shell NOT the 3d shell: Feiii+ 1s22s22phalf dozen3stwo3p63dv
One other note on writing electron configurations: A short cutting. When writing some of the lower tabular array configurations the total configuration tin can be fairly long. In these cases, you tin employ the previous noble gas to abbreviate the configuration as shown beneath. You only accept to terminate the configuration from where the noble gas leaves it:

Exceptions
As with every other topic nosotros have covered to date there are exceptions to the society of fill also. But based on the electron configurations that are generated, these exceptions are easy to empathise.
In the d block, specifically the groups containing Chromium and Copper, in that location is an exception in how they are filled.
Here are the actual configurations:
In these columns, the 4s and 3d
Practice, Practice, Do
There are lots of quizzes on electron configurations you can practice with located hither
Orbital Diagrams
Some other way to represent the guild of fill for an atom is by using an orbital diagram frequently referred to equally "the petty boxes":

The boxes are used to correspond the orbitals and to show the electrons placed in them. The lodge of fill is the same but as y'all can see from to a higher place the electrons are placed singly into the boxes earlier filling them with both electrons. This is called Hund's Rule: "Half fill before you Full fill" and over again this dominion was established based on energy calculations that indicated that this was the way atoms actually distributed their electrons into the orbitals.
Periodic Properties
One of the really cool things about electron configurations is their relationship to the periodic table. Basically the periodic table was constructed and so that elements with similar electron configurations would be aligned into the aforementioned groups (columns).

Periodic Table showing last orbital filled for each element
The periodic table shown to a higher place demonstrates how the configuration of each element was aligned so that the terminal orbital filled is the same except for the shell. The reason this was done is that the configuration of an element gives the element its properties and like configurations yield similar backdrop.
Permit'due south become through some of the Periodic Properties that are influenced directly by the electron configuration:
Atomic Size
| The size of atoms increases going downwardly in the periodic table. This should exist intuitive since with each row of the table you are adding a shell (north). What is not as intuitive is why the size decreases from left to right. But again the structure of the electron configuration gives us the answer. What are you doing as yous get beyond the periodic tabular array? Answer, calculation protons to the nucleus and adding electrons to the valence trounce of the element. What is non changing equally you cross a period? Answer, the inner shell electrons. So think of it this way, the inner vanquish electrons are a shield against the pull of the nucleus. As you cross a period and increase the number of protons in the nucleus yous increase its pull simply since you are simply adding electrons to the new shell the shield is non increasing but remains the same all the way across. This means the pull on the electrons beingness added to the valence beat out is increasing steadily all the style beyond. What happens if you pull harder on the electrons? Well, they come closer to the nucleus and the size of the cantlet decreases. The effect of the nucleus pulling on the electrons being added beyond a period is called the effective nuclear accuse and is calculated as ZEff = #protons - Core # Electrons. Then for example the pull felt by Sulfur would be ZEff = 16 - 10 = +6 | 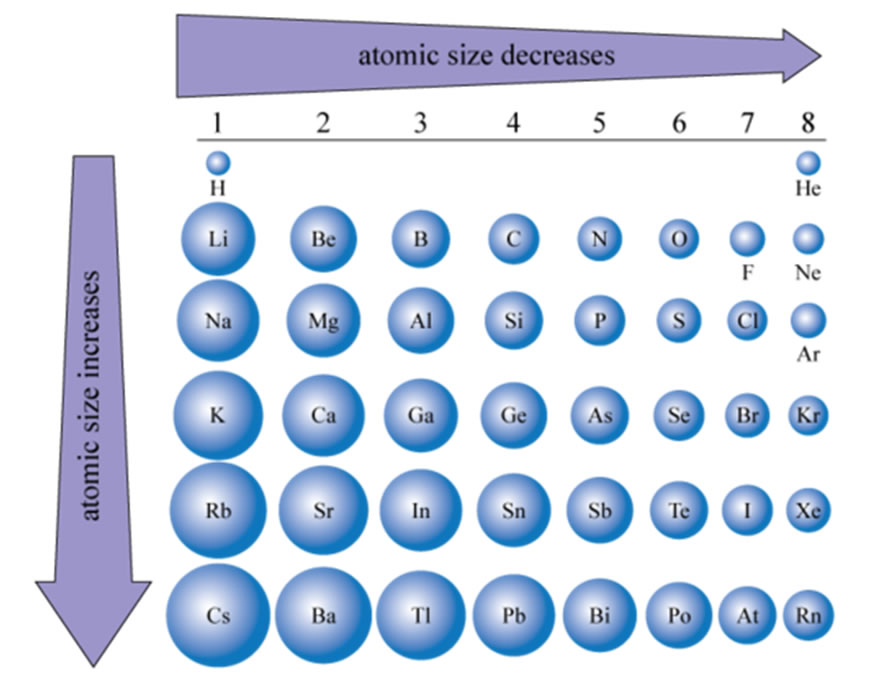 |
Electronegativity
Electronegativity may be the almost important of the periodic properties you can learn and sympathize since and so many other properties are depend on its value. Electronegativity is an atoms ability to pull electrons towards itself.
Electronegativity is more often than not expressed by the Pauling Calibration and the values were determined experimentally. The tabular array below shows the scale values for the elements.
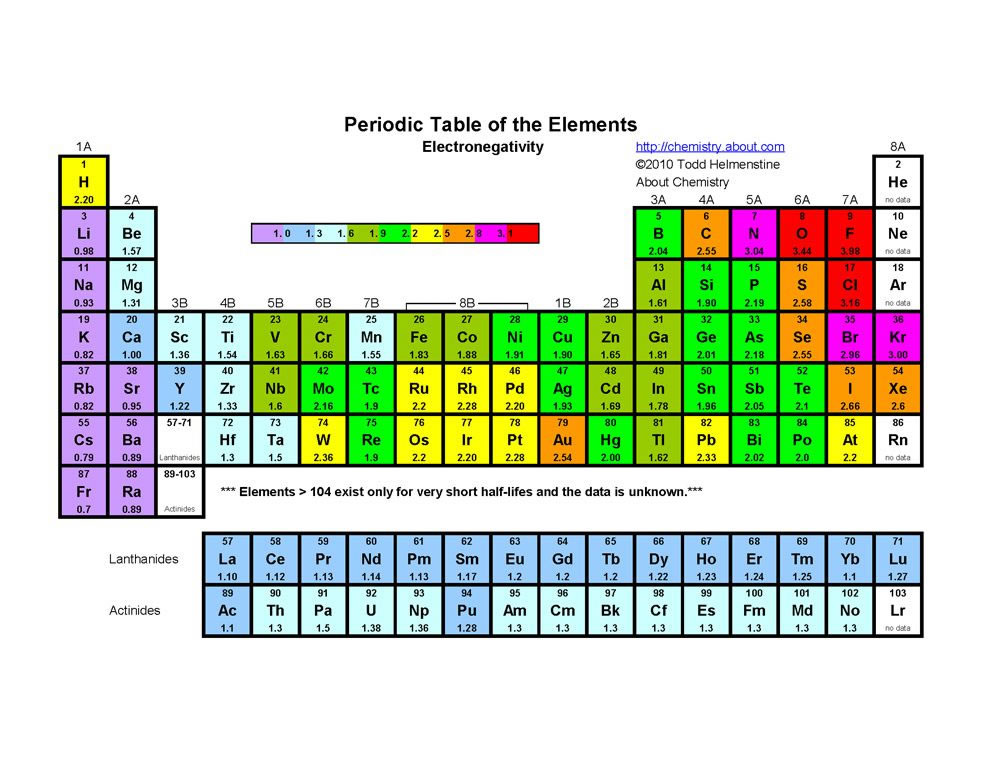
The electronegativity values increase from left to right and lesser to top in the periodic table excluding the Noble gases. The most electronegative chemical element is Fluorine.
From these electronegativity values we tin derive the patterns of 2 other periodic properties: Ionization Energy and Electron Analogousness.
| | Ionization EnergyIonization free energy is the amount of energy required to remove an electron from an atom. All ionization energies are positive values because all of these removals (even those for elements that form positive ions) require input of energy. The more electronegative the element, the higher the ionization eneregy. |
Electron AffinityThe Electron Affinity of an element is the amount of energy gained or released with the improver of an electron. The electronegativity and Electron Affinity increases in the same design in the periodic tabular array. Left to right and bottom to top. | |
Noble Gas Notation For Oxygen,
Source: https://www.chem.fsu.edu/chemlab/chm1045/e_config.html
Posted by: estradahund1935.blogspot.com



0 Response to "Noble Gas Notation For Oxygen"
Post a Comment